Thalidomide and society
The late 1950s saw the introduction of the drug thalidomide in the market, leading to one of the worst medical disasters in history. It is estimated more than 24,000 babies were born worldwide with congenital disabilities as a result. Government records reveal its far-reaching impact.
Important information
This page features documents that contain outdated and derogatory descriptions of disabled people. Original language is preserved here to accurately represent our records and to help us fully understand the past.
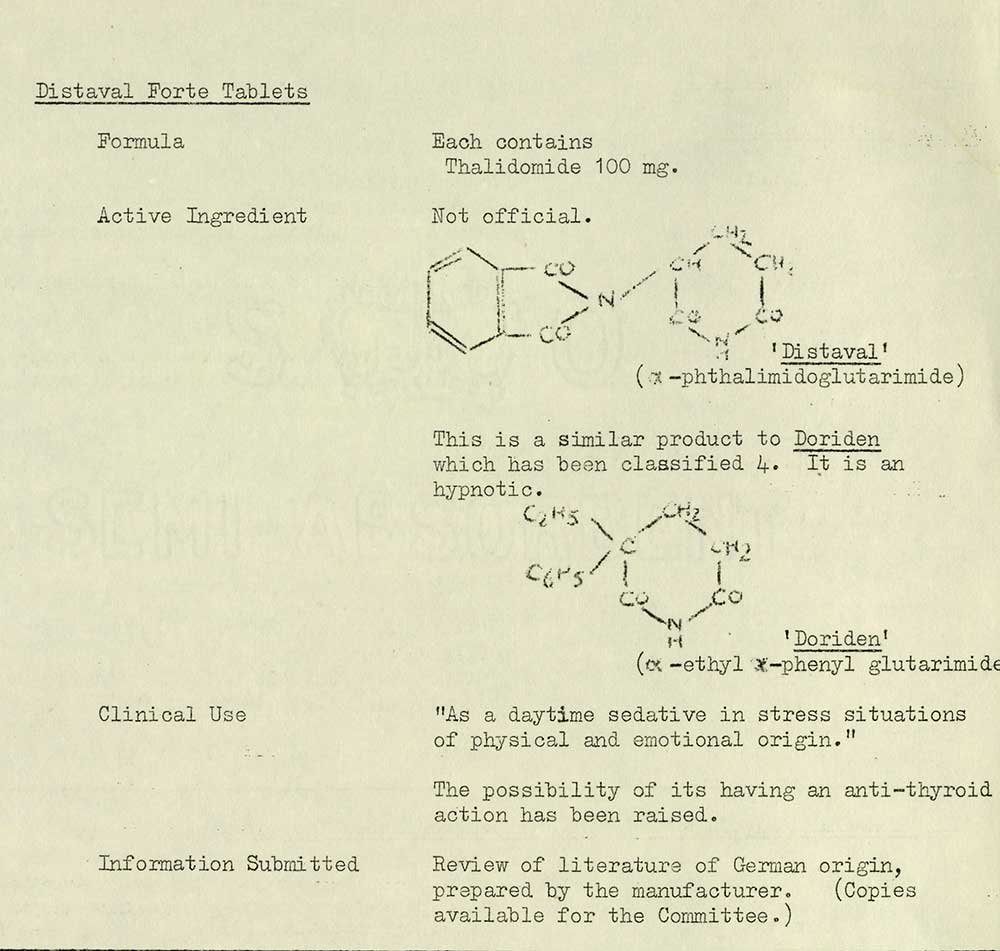
The classification of Distaval (thalidomide)
Date: 1958
Catalogue reference: View the record N/A in the catalogue
Thalidomide was introduced to the UK market in 1958 by the Distillers Company under the trade name Distaval. It was presented to the Joint Standing Committee on the Classification of Proprietary Preparations, which is where it first appears in our records. The Committee’s purpose was to classify drugs only, namely for price regulations relating to prescribing; there was no testing involved.
Distaval was indicated ‘As a daytime sedative in stress situations of physical and emotional origin’. While the Committee noted ‘the possibility of its having an anti-thyroid action’, it was also presented alongside marketing literature prepared by Chemie-Grunenthal, its German manufacturer, leading the Committee to classify the product as category N: new drug of proved value. The assessment led to it becoming exempt from purchase tax, making it more attractive for doctors to prescribe on the NHS.
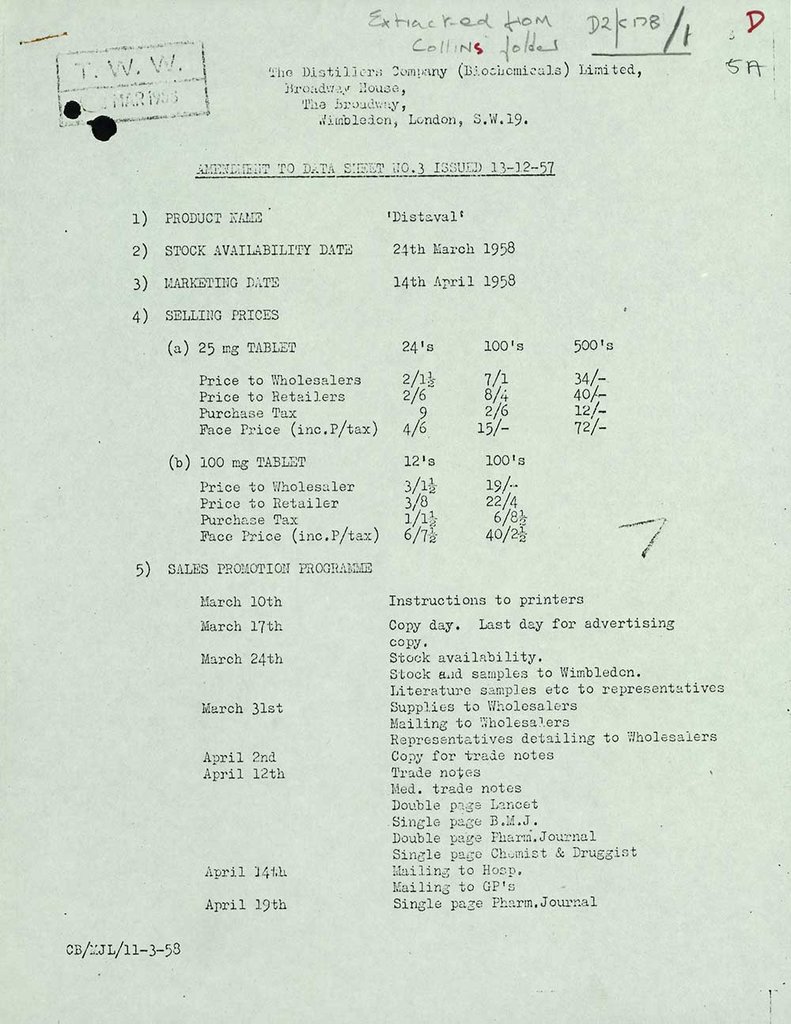
Copies of data sheets from the Distillers Company
Date: 1958
Catalogue reference: View the record N/A in the catalogue
Thalidomide was licensed for sale by Distillers (Biochemicals) Ltd in the UK and was sold under the names Distaval, Distaval Forte, Asmaval, Tensival, Valgis and Valgraine. It was prescribed by NHS and private doctors between April 1958 and November 1961 when it was withdrawn. During this time it was used primarily as a sedative and it was also given to pregnant woman to relieve the symptoms of morning sickness.
This data sheet from the Distillers Company detail thalidomide's marketing schedule in 1958. It shows that doctors were offered samples to distribute to patients. Exactly how many people were given the drug will never be known.
Marketing leaflet for Distaval
Date: 1960
Catalogue reference: View the record N/A in the catalogue
Thalidomide was marketed under the name Distaval in the UK as a ‘highly effective’ drug to ward off stress of ‘physical of emotional origin’ and to combat sleeplessness.
As a non-barbituric sedative it was promoted widely as remarkably safe. It was considered, at the time, to be impossible to overdose on. Barbiturates had a reputation for causing accidental poisonings, so thalidomide was marketed as a wonderful new alternative that could even be ‘safely given to children.’
The reality was the thalidomide, no matter the dose, could cause a range of side-effects, including damage to the nerves. In pregnancy the results could be catastrophic, causing miscarriages and stillbirths. Babies could be born with shortened or missing limbs, or damage to eyes, ears and internal organs.

Ministry of Health Circular
Date: 17 May 1962
Catalogue reference: View the record N/A in the catalogue
George Godber served as the Chief Medical Officer from 1960–1973. In May 1962 he sent this circular to doctors asking them to ensure women of child-bearing age, who may still have had access to thalidomide as a result of being prescribed it prior to its removal, were aware of the risks.
An emphasis on the brand name ‘Distaval’ was given, but notably there was no mention of the numerous other brand names it was sold under, like Tensival, Asmaval, Valgraine, and Valgis.
Godber warns that ‘unnecessary alarm’ among expectant mothers should be avoided, noting that in the warning given to the general public in the press the risk was deliberately described as ‘slight.’

Letter from Medical Officer of Health for Woking about thalidomide cases in their area
Date: 25 September 1962
Catalogue reference: View the record N/A in the catalogue
In this letter the Medical Officer of Health for Woking gives details of the thalidomide cases in their area. Letters from medical officers reveal the difficulties in ascertaining whether a mother had taken thalidomide due to incomplete, missing, or destroyed medical records as well as mothers and doctors not being able to recall the date and nature of prescriptions.
This lack of data would severely impact many families' later claims for compensation from the Distillers Company.

Letter from an MP to the Ministry of Health about a thalidomide-affected baby in his constituency
Date: 29 May 1962
Catalogue reference: View the record N/A in the catalogue
This letter is emblematic of the types of letters the Ministry of Health received in the months after the true effects of thalidomide came to light. One MP wrote to Enoch Powell, the Minister of Health, concerning the birth of a thalidomide-affected child in his constituency. He asks what action the Ministry and the Distillers were taking.
His letter also gives us insight into how physical disability could be viewed at the time, with the baby being described as a ‘monster’ with ‘frightful characteristics’.

Posters calling for the public to boycott the Distillers products
Date: 1972–1973
Catalogue reference: View the record N/A in the catalogue
These posters were pasted over hoardings for Distillers products and sent to organisations as part of a campaign designed to put pressure on the Distillers Company to increase their offer of compensation. However, since the matter of compensation was the subject of civil litigation it was noted that this could constitute a contempt of court.
The Metropolitan Police surmised that the posters were professionally produced and – not bearing the names of the printers – thus contravened the Newspapers, Printers and Reading Rooms Repeal Act of 1869.

Letter from journalist John Pilger to Alfred Morris, Minister for the Disabled
Date: 14 January 1978
Catalogue reference: View the record N/A in the catalogue
In 1968 a settlement had been made for 62 children by the Distillers. A later settlement in 1973 provided compensation for another 338 children on the so-called 'X' List, which comprised children whom Distillers accepted as children disabled as a result of thalidomide exposure. The 'Y' List comprised 98 children who had not been accepted by Distillers as thalidomide-affected children at the time of the 1973 settlement.
In March 1978 Sir Alan Marre was appointed to consider what further steps the Distillers Company should take in relation to any outstanding 'Y' List cases. The investigative journalist John Pilger brought to the fore the emotive cases of some of those on the 'Y' List, writing to politicians and publishing articles in the Daily Mirror.

Letter regarding research into thalidomide's effectiveness as a treatment for a complication of leprosy
Date: 27 August 1978
Catalogue reference: View the record N/A in the catalogue
During the mid-1960s research on thalidomide began on its effectiveness in treating a complication of leprosy called Erythema nodosum leprosum (ENL). Many felt that the risks of using thalidomide were too high.
This letter, from Henry Bunje of the Medical Research Council to Dr Crawford from the department of Anatomy and Embryology at University College London, defends the Council's position on using thalidomide for research at the Leprosy Research Unit in Malaysia in 1978.
